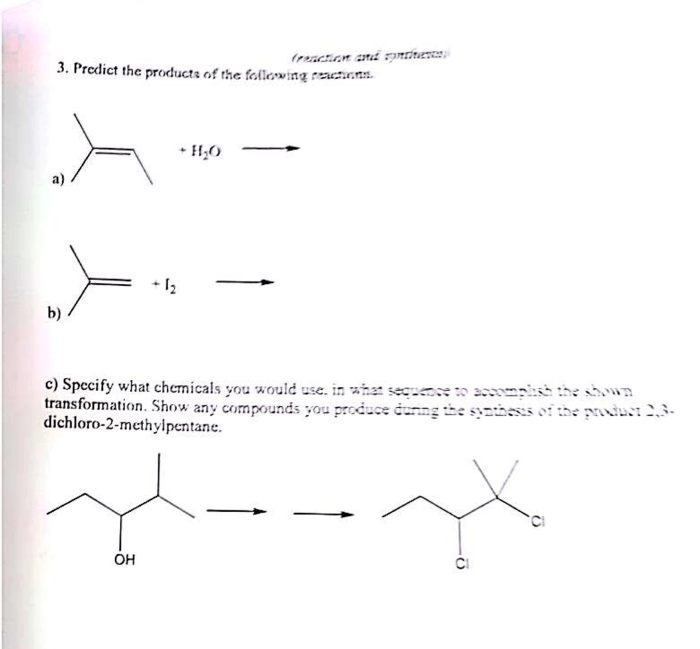
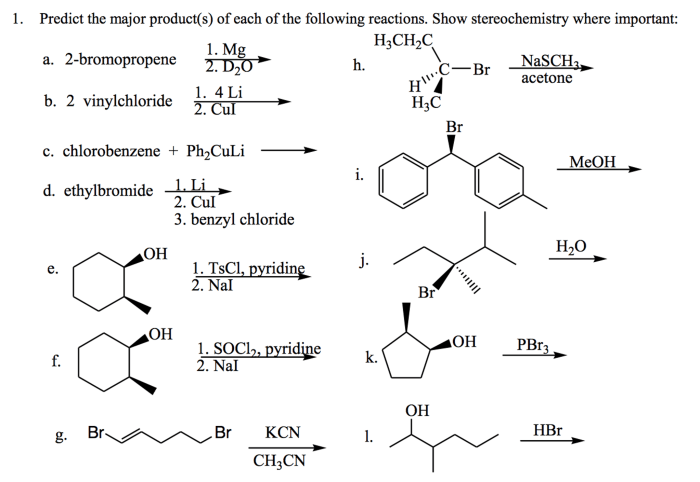
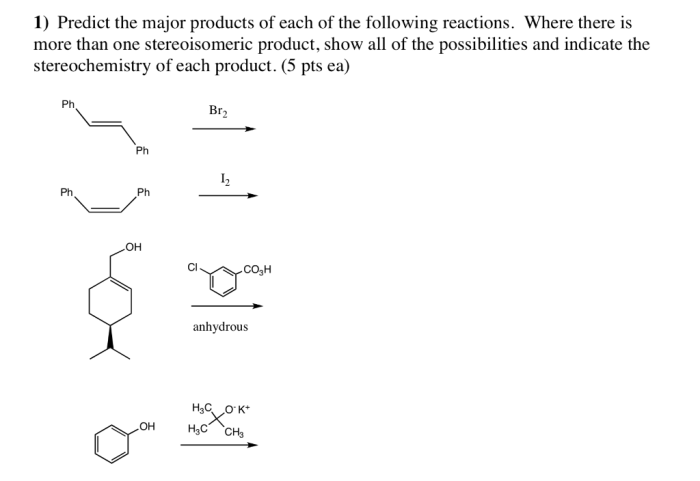
Predict the major products for each of the following reactions. This topic delves into the fascinating world of chemical reactions, exploring the intricate interplay between reactants, conditions, and the resulting products. Join us on an enlightening journey as we unravel the secrets of predicting reaction outcomes.
Chemical reactions, the cornerstone of chemistry, encompass a vast array of transformations, each governed by specific rules and principles. Understanding these principles empowers chemists to predict the products of reactions, enabling them to design and synthesize new molecules with desired properties.
Reaction Types
Chemical reactions can be classified into different types based on the changes that occur in the reactants and products. The type of reaction can significantly affect the products formed.
Some common types of reactions include:
- Combination reactions:Two or more reactants combine to form a single product.
- Decomposition reactions:A single reactant breaks down into two or more products.
- Single-replacement reactions:One element replaces another element in a compound.
- Double-replacement reactions:Two compounds exchange ions to form two new compounds.
- Combustion reactions:A substance reacts with oxygen to produce heat and light.
Reactant Properties, Predict the major products for each of the following reactions
The properties of the reactants involved in a reaction can also influence the products formed. Some important reactant properties include:
- Reactivity:The tendency of a reactant to undergo a chemical reaction.
- Concentration:The amount of reactant present in a given volume.
- Structure:The arrangement of atoms and bonds within a reactant molecule.
Reaction Conditions
Reaction conditions, such as temperature, pressure, and solvent, can also affect the products formed. For example:
- Temperature:Increasing the temperature can increase the rate of a reaction and lead to different products.
- Pressure:Increasing the pressure can favor reactions that produce fewer gas molecules.
- Solvent:The solvent can influence the solubility of the reactants and products, which can affect the reaction rate and products.
Product Analysis
After a reaction has occurred, it is important to analyze the products to determine their identity and structure. Common methods for product analysis include:
- Chromatography:Separates compounds based on their physical properties.
- Spectroscopy:Identifies compounds based on their absorption or emission of electromagnetic radiation.
- Titration:Determines the concentration of a substance in solution.
Common Queries: Predict The Major Products For Each Of The Following Reactions
What factors influence the products formed in a chemical reaction?
The type of reaction, properties of the reactants, reaction conditions, and the presence of catalysts all play a role in determining the products formed.
How can we predict the products of a reaction?
By understanding the reaction mechanism and applying knowledge of reaction types, reactant properties, and reaction conditions, we can make informed predictions about the products.
What analytical techniques are used to identify reaction products?
Techniques such as chromatography, spectroscopy, and mass spectrometry provide valuable information about the identity and structure of reaction products.